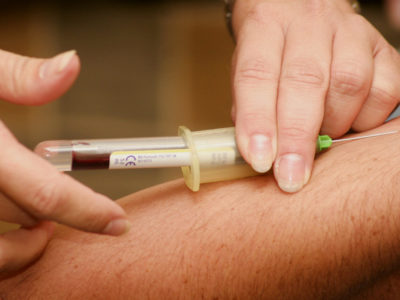
To obtain a concentrate of antibodies (immunoglobulins) against Covid-19 from the blood of an infected patient, a procedure called plasmapheresis must be performed.
This separates the corpuscular elements from the blood by centrifugation or filtration. This technique has been known and used for some time.
It was invented by Drs. Abel and Rowntree in 1913 and then developed by Dr. Lucas in 1950. Even before this period, a rudimentary method for extracting serum (rich in immunoglobulins) from the blood of infected patients for therapeutic use was already known. . In 1890, Von Berhing and Kitasato demonstrated for the first time that blood serum had to contain substances capable of fighting infection if it originated in the blood of a patient infected with that infection.
Therapeutic use of plasmapheresis to obtain concentrations of plasma immunoglobulins (hyperimmune plasma) has also recently been done to combat the Ebola epidemic of 2013 and the Sars-CoV-1 of 2003. It should be noted that the use of plasma in diseases such as Aidso , the normal flu did not bring benefits.
The use of plasma for the treatment of Sars-CoV-2 (Coronavirus, ed) is somewhat debated in the scientific community and has already been published in several journals such as Lancet. In China, the first experiments date back to January 20, 2020. In Italy it was recently chosen in the hospitals of Pavia and Mantua. The results were immediately encouraging. The therapeutic effect is rapid and without significant side effects, except in cases of receptor hypersensitivity to the plasma components of another individual or imbalance in the coagulation processes, which have already been compromised by Covid-19.
Unfortunately, however, obtaining large amounts of plasma with coronavirus-active immunoglobulins is not easy. Applicants must be patients who have had the disease and have fully recovered from Sars-CoV-2. They should not have other infections (hepatitis and HIV, for example) and they should not have had serious antibody reactions in their life. They must be women who have not had pregnancies because pregnancy creates a complex system of antibodies that can react to the recipient.
There must be all the requirements established by law that serve to protect donors and transfusions. Each donor can donate a maximum of three times and their plasma can help a maximum of two patients. The result is that we do not have as many possible donors as they might need. Numbers that do not fit, therefore, large-scale applicable therapies. Also, we don’t know how effective the antibody response is over time. Finally, when it comes to cost, to understand, this is not very high, but you certainly can’t talk about zero cost therapy. Everything else is fake news.
Some additional considerations: since plasma infusion with concentrated (or hyperimmune) immunoglobulins represents emergency therapy only, it would be necessary to study and understand which antibodies are effective, isolate them, purify them and then administer only those in controlled and pharmacological doses. As with tetanus immunoglobulins, for example. A procedure that transforms what is now an immunoglobulin donation into a real pharmaceutical product.
Finally, it should be noted that plasma donation with concentrated (or hyperimmune) immunoglobulins has nothing to do with the creation and use of monoclonal antibodies, such as the recently identified one capable of neutralizing the SarCov2 virus described in the journal Nature Communications. In this case, researchers from the University of Utrecht produced the 47D11 antibody by obtaining it from ‘chimera’ antibodies, that is, derived from human and runner cells.